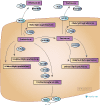
Substance abuse disorders are a significant public health cost [PMID: 34453125]. Prescribers have limited choices for pharmaceutical therapies with only three FDA-approved choices for alcohol use disorder and zero for cocaine use disorder. Disulfiram is an FDA-approved treatment for alcohol use disorder which is also used for cocaine use disorder. There are some preliminary studies on the PGx of disulfiram but little replication.
PharmGKB together with Dr Aneysis De Las Mercedes Gonzalez (Stanford University School of Medicine), have published PharmGKB summary: disulfiram pathway in Pharmacogenetics and Genomics, 2023 Sep 21. doi: 10.1097/FPC.0000000000000509. Online ahead of print. PMID: 37728645. It summarizes the candidate genes involved in disulfiram PGx in pharmacokinetics and action in dopaminergic, seratonergic and noradrenergic neurons and highlights the knowledge gaps.
As always interactive versions of the diagrams with underlying linked evidence, are available on PharmGKB:
Disulfiram Pathway, Pharmacokinetics,
Disulfiram Pathway, Pharmacodynamics (Cocaine and Ethanol PK),
Disulfiram Pathway, Pharmacodynamics (Dopaminergic neuron),